Product description
According to《Regulations for the Administration of Veterinary Drug Registration》 Article 3 Veterinary drug registration means that a veterinary drug registration applicant (hereinafter referred to as the applicant) applies for new veterinary drug registration, imporing veterinary drug registration, re-registration and supplementary registration for marketing authorization of veterinary drugs in accordance with legal procedures and relevant requirements. The Ministry of Agriculture and Rural Affairs organizes relevant professional and technical institutions to review the safety, effectiveness and quality control of the proposed veterinary drugs based on laws and regulations and existing scientific knowledge, and decide whether to approve the application.
Article 5 The applicant to apply for veterinary drugs within the territory of registration shall be handled in accordance with the procedures and requirements of the new veterinary drug registration, foreign applicants applying for import of veterinary drug registration in accordance with the procedures for imported veterinary drugs registration and registration.
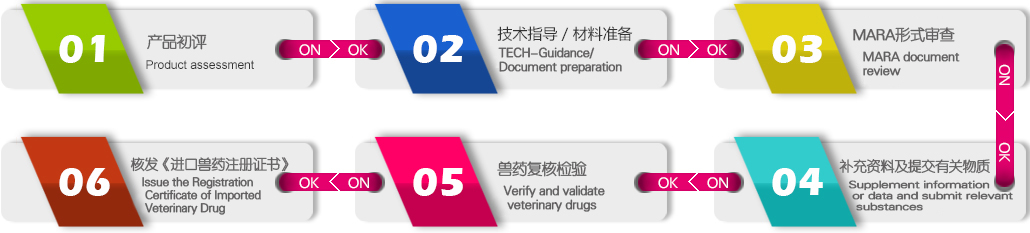
We provide you with the following professional services:
1. Product evaluation, and compliance guidance.
2. Guide and assist in the preparation, previewing, preparation and submission of registration materials.
3. Assist to submit sample for validation and verification;
4. Registration progress tracking and result querying.